
SK pharmteco 2024 Continuous Flow Symposium
SK pharmteco, the world leader in continuous flow processing, is hosting a symposium for customers and key contacts entitled ‘Continuous
SK pharmteco offers integrated CDMO services across Cell and Gene Therapy, Small Molecule, and Analytical Services for drug substances and products
Built on 80 years of experience, SK pharmteco is a trusted partner specializing in the manufacture of APIs and intermediates, cell and gene therapy technologies, registered starting materials and analytical services for the pharmaceutical industry worldwide. Our global operations have the capability and capacity to support your needs from development through commercial production.
Our complementary assets and expertise enable us to provide the highest quality services to our customers.
SK pharmteco specializes in the commercial and clinical production of APIs, Advanced Intermediates, Registered Starting Materials, Key Building Blocks and Viral Vectors. Our offerings include process R&D, analytical method development and stability, scale-up and optimization, validation and commercial production. Additionally, we offer extensive Adeno-Associated Viral (AAV) and Lentiviral vector development and cGMP production capabilities.
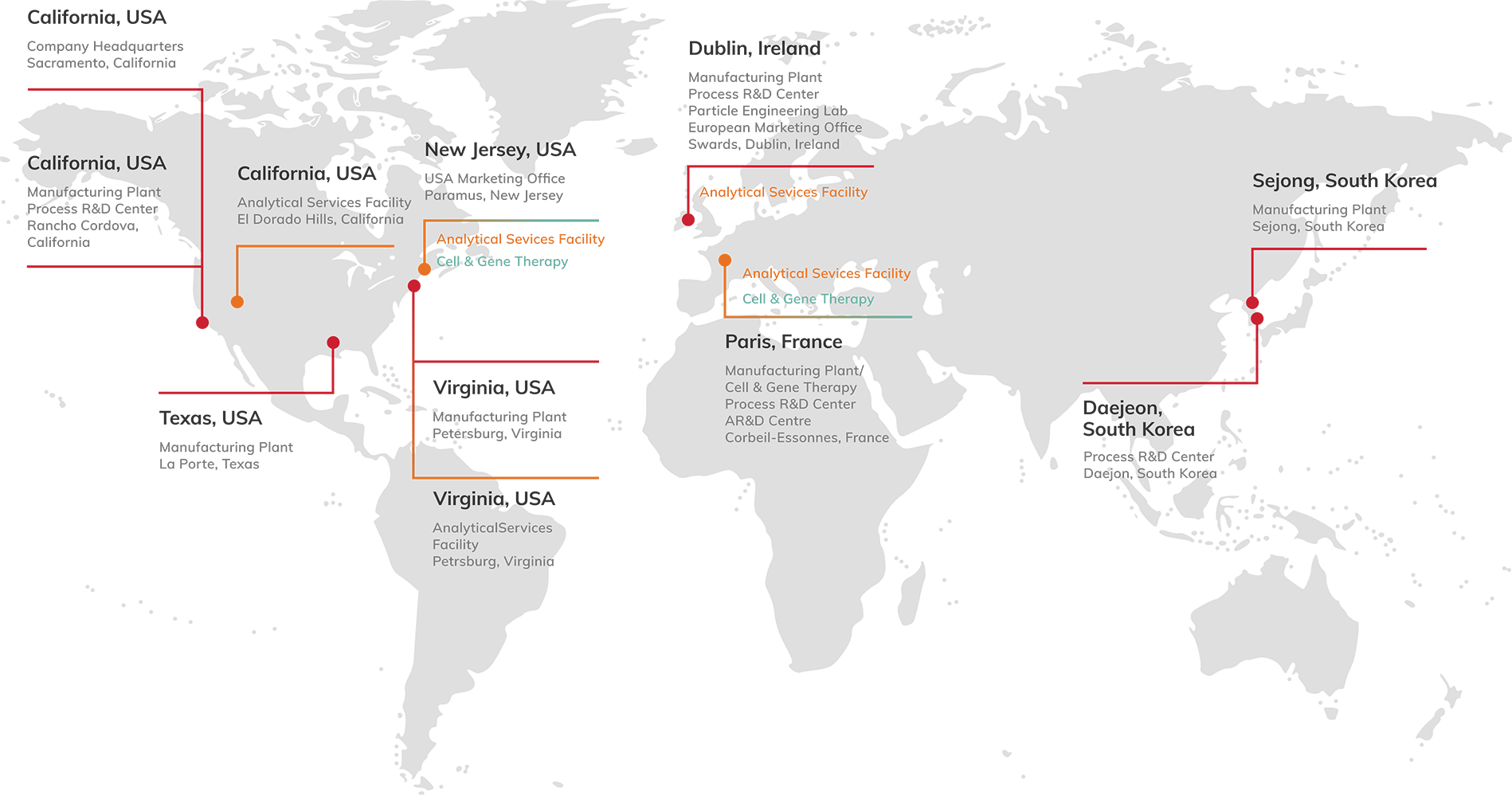
Our global operations include 7 cGMP manufacturing and development facilities across the United States, Korea, Ireland and France. We also operate dedicated analytical services facilities.
SK pharmteco is a leader in operational excellence and continuous improvement. Our highly skilled team delivers innovative solutions utilizing advanced technologies:
We provide PAI and full regulatory support as well as complete confidentiality. We have a strong record of environmental, health, and safety performance. All our plants have been successfully audited by global regulatory agencies including the FDA (US), EMA (Europe), PMDA (Japan), and MFDS (Korea).
With a strong technology toolbox and ~1,000 m³ (~265,000 gal) of global small molecule capacity and two facilities dedicated to large molecules, we have the capability and capacity to support your needs across the full lifecycle.
The most trusted global partner in the delivery of innovative medicine
We are committed to changing the world through our unwavering dedication to life-saving therapies.
We enable our partners with cutting-edge technology, expertise and customer centricity, allowing them to bring breakthrough treatments to patients and delivering happiness and health to stakeholders everywhere.


Diazomethane, Azide, Hydrazine, Ozone
Fixed bed, CSTR, PAT
Process Development, CGT Analysis, Viral Vector Development & CGMP Manufacturing, Plasmid DNA Manufacturing, & Cell Therapy Manufacturing
Simulated Moving Bed (SMB), Batch
Up to 4 m3 (~1,000 gal) scale
Down to 10 ng/m3 containment
Schedule II-V Manufacturing
Schedule I By Request
Crystallization, Micronization

SK pharmteco, the world leader in continuous flow processing, is hosting a symposium for customers and key contacts entitled ‘Continuous

Biopharmaceutical contract manufacturer SK pharmteco commits to production, testing, and release of Ferring’s U.S. approved Adstiladrin® (nadofaragene firadenovec-vncg) The deal

We are pleased to announce that Julie Osborne has joined SK pharmteco as VP, Global HR. Julie has held several

"*" indicates required fields