About Us
Who We Are
SK pharmteco supports all analytical requirements for pharmaceutical intermediates, Active Pharmaceutical Ingredients (APIs), Cell and Gene Therapy (CGT), and formulated drug products. From Analytical Method Development and Implementation to Analytical Method Validation and stability, SK pharmteco is fully equipped with fully compliant CGMP instrumentation geared toward product analysis and product release. SK pharmteco supports spectroscopy, chromatography, particle size distribution, calorimetry, osmolality, mass spectrometry, and ICH Stability project requirements.
SK pharmteco manufactures cell and gene therapy and active pharmaceutical ingredients (APIs) and registered intermediates for customers in the pharmaceutical industry. Our locations are worldwide, optimizing logistical and supply chain performance excellence.
Global Presence
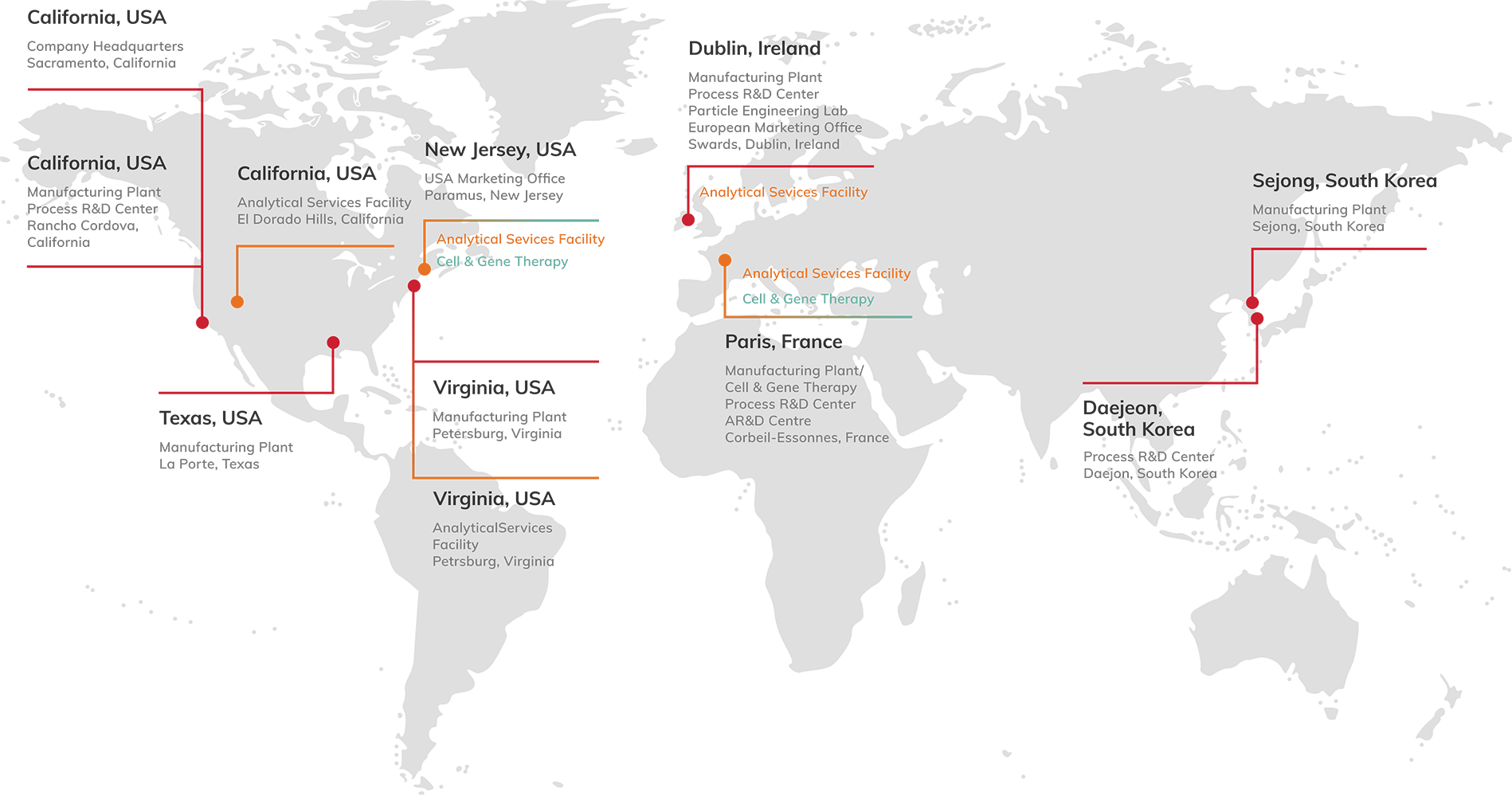
USA
California, USA Company Headquarters
Manufacturing Plant
Process R&D Center
Analytical Services Facility
Texas, USA Manufacturing Plant
Virginia, USA Manufacturing Plant
Analytical Services Facility
Pennsylvania, USA Cell and Gene Therapy Manufacuring
Analytical Services Facility
EUROPE
Dublin, Ireland Manufacturing Plant
Process R&D Center
Particle Engineering Lab
Analytical Services Facility
Corbell-Essonness, France Cell and Gene Therapy Manufacuring
Analytical Services Facility
ASIA
Daejeon, South Korea Process R&D Center
Sejong, South Korea Manufacturing Plant
With eighty years of experience in mastering challenging chemistry, we have developed capabilities that support a wide range of processes and technologies during that time. All of SK pharmteco’s products are manufactured in full compliance with the U.S. Food and Drug Administration’s (FDA) current Good Manufacturing Practices (CGMP) and other regulatory agencies.
See our services, submit a sample or request a quote now!

