
SK pharmteco is a global leader for pharmaceutical intermediates, Active Pharmaceutical Ingredients (APIs), and formulated drug products, Cell and Gene Therapy (CGT), and analytical services.
In the entries below, we’ll share our thoughts and comments on these areas and other related topics. Please feel free to contact us to suggest topics of interest.

Comprehensive and Accelerated Testing Programs for Cell and Gene Therapies
SK pharmteco’s Cell and Gene Therapy Analytical Testing Services are readily available and positions our clients to successfully meet the challenges associated with commercializing cell and gene therapies. We offer full testing programs that can be customized to specific products and modalities. A complete package of platform assays meets the full spectrum of global regulatory requirements and are located at a single provider. Consolidating testing labs allows for the acceleration of results and reduced sample volumes required for testing. By having the platform assays, this significantly speeds the time required for Analytical Implementation and GMP batch release with no wait times. SK pharmteco’s platform assays

Keys To Effective Method Development
Effective method development is crucial for the quality control of Active Pharmaceutical Ingredients (API) and Drug Products (DP). Thorough method development enables successful downstream method validation. The regulatory guidance specifies that: Method development and validation vary by application (quantitative, qualitative, etc.). It is phase appropriate. The client may provide additional guidance/validation criteria. The validation guidance directs how AMD (analytical method development) is conducted. Early Adoption of Forced Degradation Analysis It is recommended that forced degradation be performed early in the method development lifecycle and that method parameters are suitable for mass spectrometry. This will prevent many issues that could occur in later stages and ensures
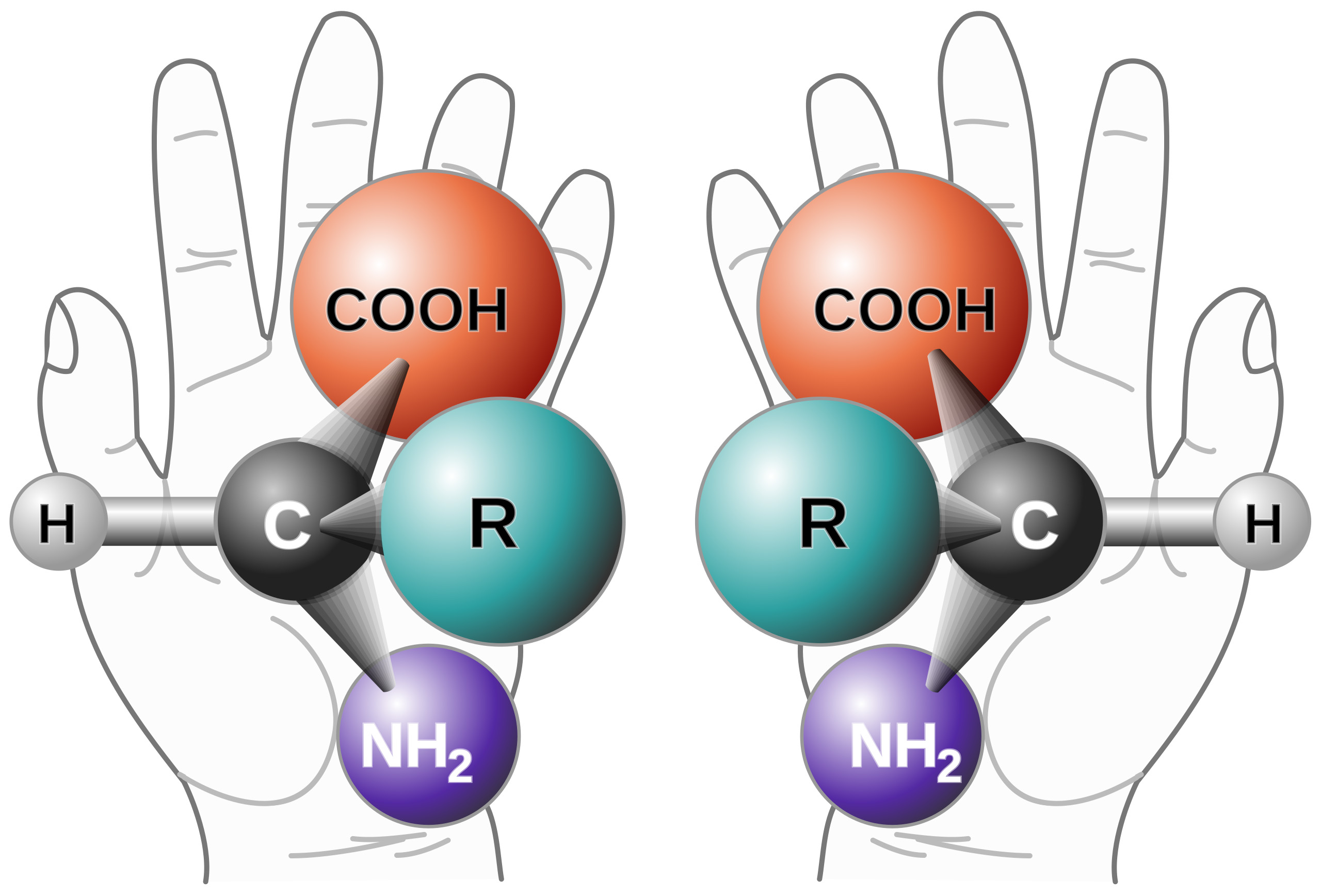
Chiral Purity Analysis – Know What Both Hands Are Doing
Background on Chiral Purity Chirality refers to the phenomenon that occurs when a mirror image cannot be superimposed. It is sometimes called “optical rotation”. The origin is from the late 19th-century Greek word kheir (‘hand’) and is one of the easiest demonstrations of the concept. Although a person’s hands may appear virtually identical, if they were switched, the outcome would be very different. Amino acids and sugars are the chiral building blocks of larger molecules such as peptides, proteins, and nucleic acids. Therefore, those polymers, in turn, are chiral as well.1 Molecules with chiral centers may have a different therapeutic impact, and this guides the
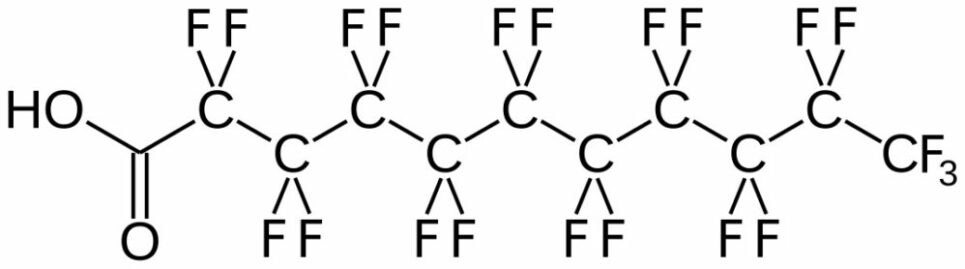
Some Background and Concerns About PFAS
The Background and Concerns of PFAS (Per- and) PolyFluoroAlkyl Substances (PFAS) are a class of ubiquitous chemicals that have been found in water, air, fish, and soil across the nation and worldwide. Known as “Forever Chemicals,” there are thousands of different PFAS, and they are present in consumer, commercial, and industrial products.1 Having one of the strongest bonds in organic chemistry, their structures proved to be resistant to heat, water, oil, and degradation.2 They are found in “food packaging and non-stick cookware, cosmetics, waterproof and stain-proof textiles and carpet, aqueous film forming foam (AFFF) to fight Class B fires, and as part of metal plating

Shedding Light on Photo-Stability Forced Degradation
Forced Degradation is the testing of a drug product or drug substance using situations more taxing than those if conditions were simply accelerated. This type of stability testing is designed to demonstrate various degradation pathways within the product or substance and assists with developing the product itself and its packaging. Within forced degradation testing, a subset of studies is known as photo-stability. The International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) and the FDA outline the guidelines. As the ICH Topic Q1B document states, “The intrinsic photostability characteristics of new drug substances and products should be evaluated to demonstrate that,

