Keys To Effective Method Development
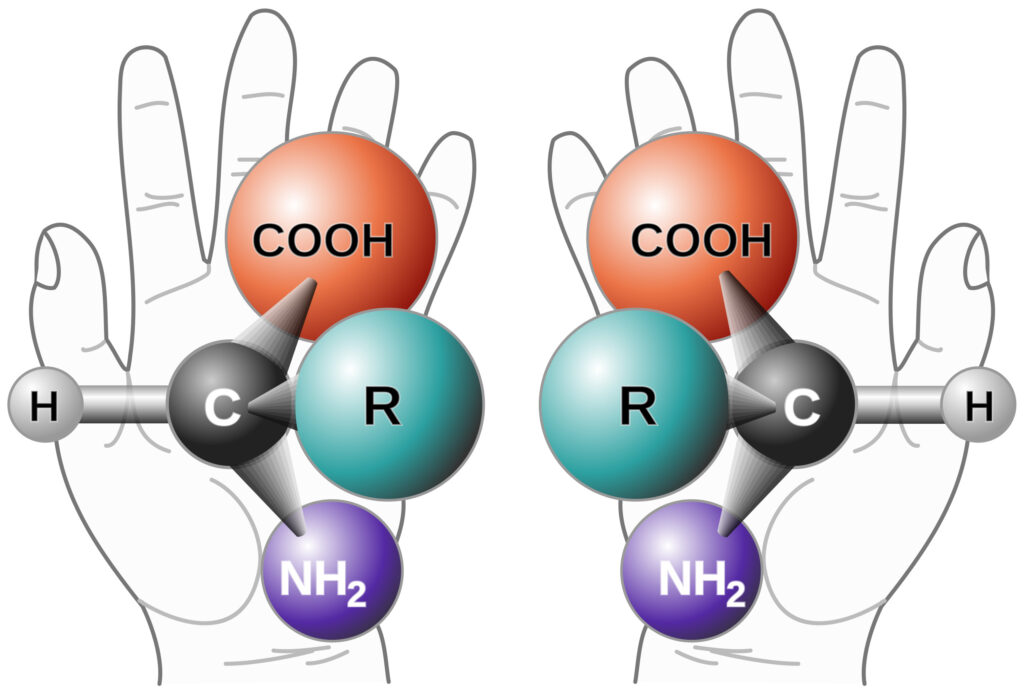
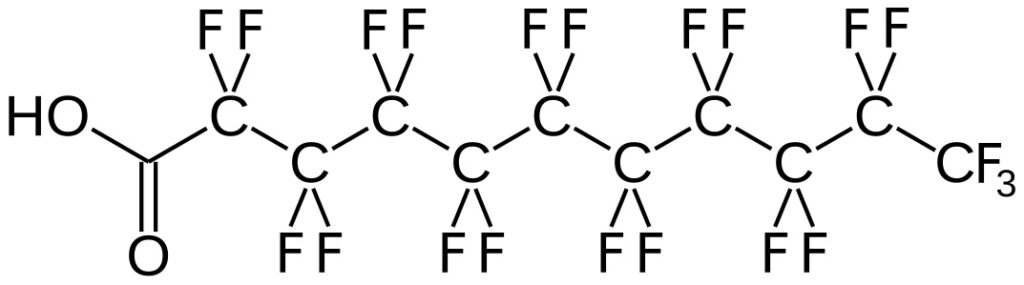
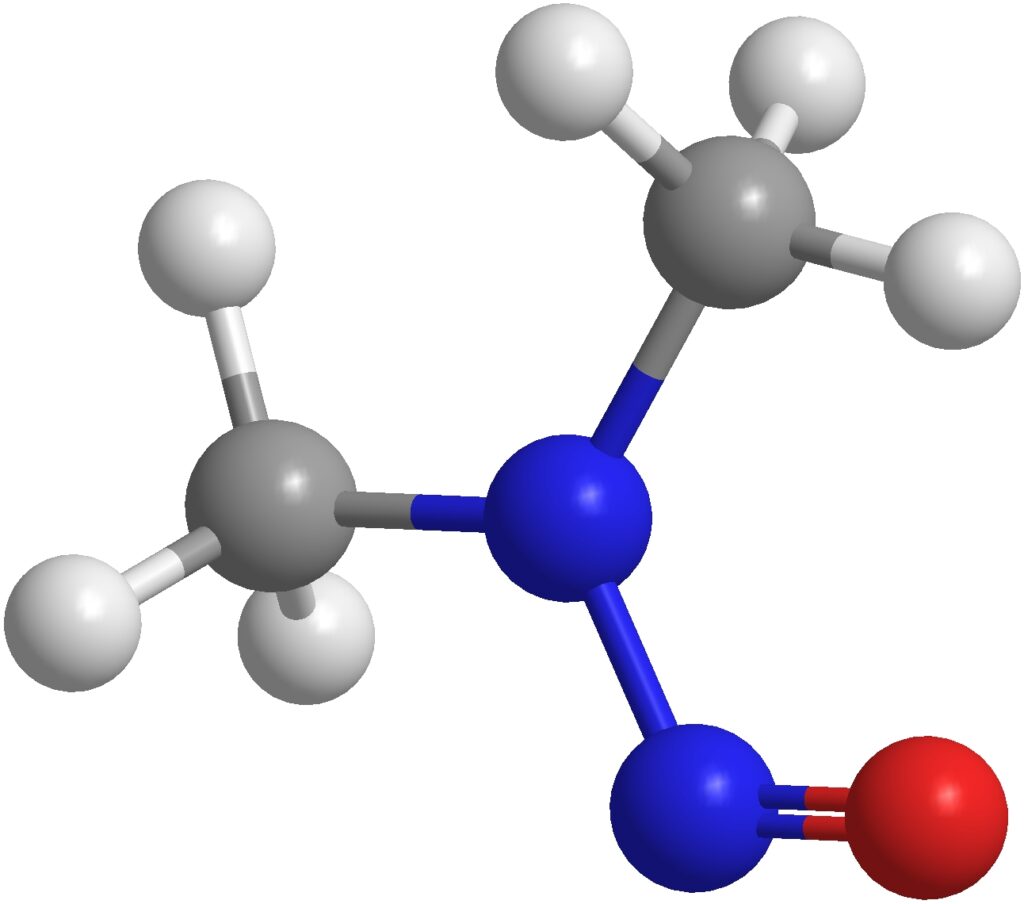
Effective method development is crucial for the quality control of Active Pharmaceutical Ingredients (API) and Drug Products (DP). Thorough method development enables successful downstream method validation. The regulatory guidance specifies that: Method development and validation vary by application (quantitative, qualitative, etc.). It is phase appropriate. The client may provide additional guidance/validation criteria. The validation guidance […]
Keys To Effective Method Development Read More »