The Korean CDMO’s new viral vector platforms can significantly save the production time and cost of AAV and lentiviral vectors
By Ji-Hyun Lee
SK pharmteco Co., South Korean conglomerate SK Group’s contract development and manufacturing organization (CDMO), has recently introduced two new viral vector platforms that are expected to save the time and cost of manufacturing innovative cell and gene therapies (CGT).
The company said on Wednesday that it launched two viral vector manufacturing platforms called LentiSure and AAVelocity in June and November, respectively.
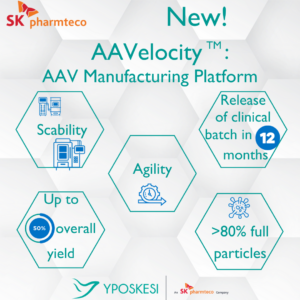
AAVelocity is “a plug-and-play suspension platform” to produce adeno-associated virus (AAV) vectors, and it can “provide a 12-month turnaround on large volume programs,” explained the company, adding that it lowers “the industry average standard bioprocessing timeframes by at least six months.”
LentiSure is a platform to produce lentiviral vectors, which is expected to significantly speed up the lentiviral vector production time.
“We will continue to expand our portfolio of development and manufacturing platforms as our commitment to lowering the cost of goods sold for our client, thereby expanding patient access to these therapies,” John T. Lee, the global head of Cell and Gene Therapies at SK Pharmteco, said in a statement.
“Future offerings in cell lines, plasmid DNA, and expanded manufacturing capacity and capabilities will further democratize access for commercial therapies and de-risk scaling for clinical programs.”
KEY TO CGT
Viral vectors are vehicles to deliver genes into target cells and the entire body, the key step in gene therapy. They are considered the most effective means of gene transfer to modify specific cell types or tissues and can be manipulated to express therapeutic genes.
AAV and lentiviral vectors are the widely used platforms for gene delivery for gene therapies and cell-based immune-oncology, which is highly sought after in line with the rapid expansion of the CAR-T cell therapy market.
Both AAVelocity and LentiSure are developed by SK Pharmteco’s European CGT clinical and commercial viral vector manufacturing subsidiary Yposkesi. The Korean parent acquired the Paris, France-based company in 2021.

Saving time and costs to produce viral vectors is significant in gene therapies that are in growing demand.
According to Evaluate Pharma, a global pharmaceutical industry tracker, the global CGT market will grow at a compound annual growth rate (CAGR) of 50% from 2022 to 2027.
The US Food and Drug Administration has already introduced a set of guidelines to expedite the approval of novel CGTs.
In line with the growing CGT market, the global viral vector market is also forecast to grow to $12.8 billion in 2028 at a CAGR of 18% from 2023, according to the global research firm MarketsandMarkets.
However, the biggest hurdle to the development and manufacturing of cell and gene therapies is their high manufacturing and R&D costs that increase the life-saving gene therapy cost burden on patients.
According to SK Pharmteco, the cost of goods and manufacturing of gene therapy can be between $500,000 and $1 million, excluding costs for R&D and the costs to run crucial clinical trials.
RELIABLE CGT CDMO PARTNER
The Korean CDMO company expects its new viral vector platforms will eventually contribute to lowering patients’ financial burden by enabling CGT developers to save their cell and gene therapy development and manufacturing costs.

As a major active pharmaceutical ingredient (API) and advanced intermediate CDMO, SK Pharmteco can also offer its CGT-developing customers a one-stop drug mass-production solution, as well as development and clinical solutions, including early process development and modular cleanrooms.
Its expanded global footprint is also a plus to its customers.
On top of Yposkesi, the Korean CDMO took over a controlling stake in the Pennsylvania-based Center for Breakthrough Medicines (CBM) this year.
With them under its wings, SK Pharmteco can allow its biotech customers to access more diverse CGT manufacturing and testing facilities and technologies, including good manufacturing practice (GMP)-certified facilities with cross-functional support in advanced analytical development.
The company also provides its customers with complete cell banking facilities and testing services for their GMP products.
Its innovative cell lines and manufacturing platforms for AAV vectors allow it to produce 100 batches of viral vectors for four clinical phase programs.
Combined with Yposkesi and CBM’s manufacturing operation systems, SK Pharmteco’s total CGT production facility site would reach 800,000 square feet in 2026 when CBM is due to complete the world’s largest single CGT manufacturing site with more than 700,000 square feet.
Yposkesi is also preparing for mass production at its second facility in 2024.
SK Pharmteco, wholly owned by SK Group’s holding firm SK Inc., was officially launched in 2019 by SK as the group’s CDMO unit.
It currently operates seven production facilities in the US, Europe, and South Korea, as well as five R&D centers worldwide.
More here: SK Pharmteco launches new viral vector platforms, key for CGT

